
Last month we talked about deciding what samples need to be collected for the day. After I go through the schedule, plan out my day, and determine what samples I need to collect, I read micro. What I mean by “read micro” is that I look at the HLP (Hsu Lactobacillus Pediococcus) tubes I have incubating and see if anything has grown.
Let’s back up a bit before I get too far ahead of myself.
What exactly is HLP?
Hsu Lactobacillus Pediococcus is a selective media that supports the growth of Lactobacillus and Pediococcus. Lactobacillus and Pediococcus are the two most common beer spoilers, so if you’re just getting into micro testing in your brewery, it’s a good one to start with. Eventually you will want a variety of general culture and wild yeast media since the only thing you can count on is the unpredictability of unwanted microbes.
Enjoy drinking sour, ropy, buttery beer? Maybe the sour, but I’ll pass on the other two. There’s also good sour and bad sour. You’ll know the difference when you’ve had a well made beer intentionally soured and one that is infected. It sure won’t be true to brand.
Luckily, no pathogens are known to exist in beer. Unfortunately some types of bacteria can make for a very unpleasant drinking experience. Lactobacillus and Pediococcus are both Gram positive lactic acid bacteria and if present can sour your beer, produce diacetyl, and affect the mouth feel.
Gram positive bacteria will stain purple when exposed to a series of dyes and a de-colorant during a Gram stain. It is used to split bacteria into two broad classifications (positive or negative) based on the cell wall. You will need a microscope with a 100X objective to see the stain and cell morphology. Lactobacillus will form rods and Pediococcus cocci or spheres.
Starting HLP
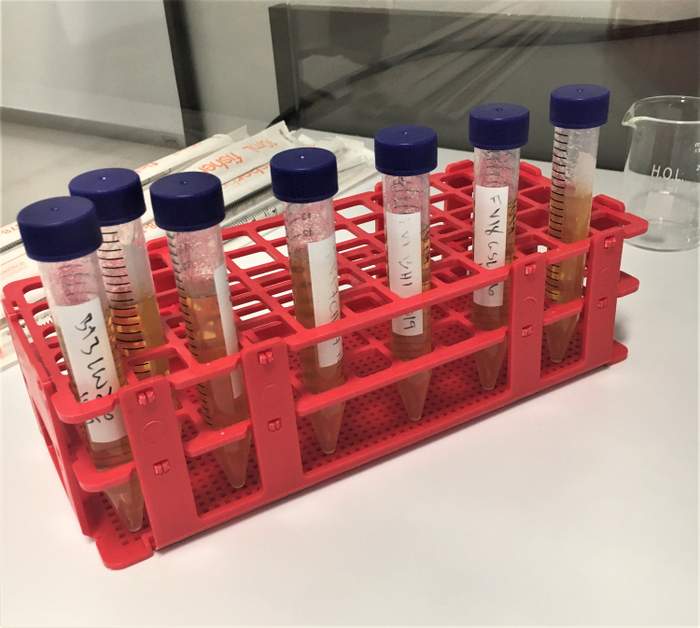
Many breweries start out using HLP since you don’t need an autoclave or anaerobic incubator or chamber to use it. You will need a constant temperature of 28-30°C to incubate your samples but since it’s a semisolid media it forms an anaerobic environment in the test tube when mixed.
To start using HLP media you will need:
- Hot plate with stirrer
- Mgnetic stir bar
- 250-500mL media bottle
- Weigh boats
- 15mL sterile test tubes
- HLP Media
- Distilled water
- Sterile 10mL pipette/bulb
- Alcohol lamp
For safety you’ll want a respirator, face shield and gloves. I work in a chemical fume hood while using it. You’ll want to check the Safety Data Sheet before working with it as it contains Cycloheximide, which is toxic, to inhibit yeast growth. Pregnant women should not use it.
HLP comes as a powder and you mix 7 grams for every 100 mL of distilled water. I usually make triple batches so I’m adding 300 mL of distilled water (which I buy at the grocery store for $0.99) to a 500 mL media bottle then 21 grams of media.
I pop in a stir bar and set it on my hot plate. I screw on the cap slightly but not all the way and wait for it to boil. It should boil for two to three minutes. I then take it off my hot place and use a sterile 10 mL pipette and bulb to transfer ~10mL of liquid media to sterile test tubes. I do all of this in a chemical fume hood.
You can reuse glass test tubes sterilized in an autoclave or buy sterile disposable 15 mL centrifuge tubes. Once made, HLP media can be used as soon as it cools to 40° C or it can be stored in the fridge for up to two weeks. It will solidify in the fridge, so after I determine how many test tubes I will need for the day, I liquefy them in a beaker of water on a hot plate.
When it’s time to inoculate HLP tubes, I use sterile, individually wrapped 10 mL serological pipettes to pull up one mL of sample and dispel it into the liquefied HLP tube. I then invert it two to three times and tap it against my thumb to get some of the bubbles out. While I’m working, I leave an open HLP tube out as a negative control. I work under a laminar flow hood but you can use an alcohol lamp or propane torch to create a sterile work environment.
Plating samples
Whenever plating samples, make sure you have a positive and a negative control. If there’s growth in my negative sample, some sort of contamination got in at some point. It could have been when I made the HLP tubes or while plating new samples. If there’s growth in some of the other tubes, I won’t know if it’s from a contamination I introduced, or from the beer. I will have to re-sample and re-test to know for sure.
For my positive control, I add a tiny bit of grain dust that collects on the bottom of the mill to an HLP tube and use the lactobacillus naturally found in malt as a positive control. If nothing grows in the positive control sample, you can’t trust your results here either. If the lactobacillus you intentionally put in your sample doesn’t grow, bacteria that you’re looking for in your samples might also not be able to grow. This could be how you made the media, the temperature of the tubes when you inoculated your sample or the wrong incubation temperature.
HLP tubes are typically read at day three and seven. I found when I was doing it this way I was forgetting to check some sample sets on day three or seven so I just started looking at all my tubes every day. My positive controls will start to show signs of growth within a day so if there is a high infection I might catch it quicker. The earlier you know there’s something unwanted growing in there, the better.
Lactobacillus is supposed to look like an inverted tear drop and Pediococcus a snowball or comet shape. My positive controls usually look like little tornadoes. For further identification you can use a Gram stain to determine if the colony is Gram positive or negative and look at the shape of the bacteria. Catalase and oxidase tests will also help with identification.
So what do you do if you see growth? One to two colonies doesn’t necessarily mean your beer will be affected, it also doesn’t mean it won’t.
Amy Todd is the Owner and Analyst for Zymology Labs as well as a lab manager for Zero Gravity Craft Brewery in Burlington, Vt.




Leave a Reply
You must be logged in to post a comment.